Thyroid Hormones and Fetal Brain Development
Mar 24, 2025
Rita Singha
Pregnancy Health
Thyroid hormones play a key role in fetal brain development, especially during early pregnancy. Here's what you need to know:
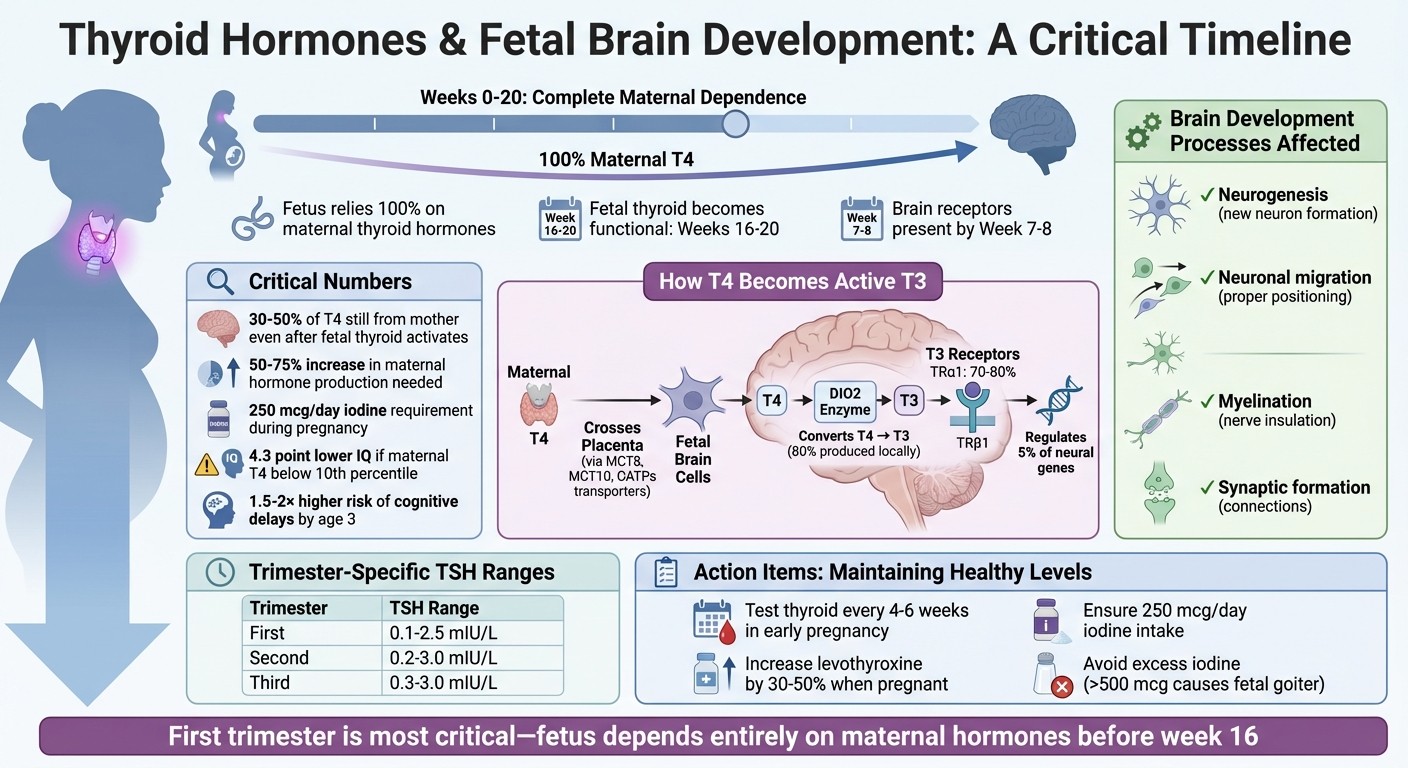
The fetus depends entirely on maternal thyroid hormones until its thyroid becomes functional around weeks 16–20.
Maternal thyroid hormones influence critical processes like neurogenesis, neuronal migration, myelination, and synaptic formation.
Low maternal thyroid hormone levels, particularly in the first trimester, can lead to structural brain changes and cognitive challenges in children, such as lower IQ scores and a higher risk of developmental disorders.
Iodine deficiency is a common cause of thyroid issues during pregnancy, making adequate iodine intake (250 mcg/day) essential for both maternal and fetal health.
Regular thyroid function testing and proper management of thyroid conditions (e.g., hypothyroidism) during pregnancy are crucial to ensure healthy brain development.
Maintaining healthy thyroid levels during pregnancy is critical for your baby's brain development. Early monitoring, sufficient iodine intake, and appropriate thyroid hormone treatment can help prevent long-term developmental issues.
How Thyroid Hormones Support Fetal Brain Development Timeline and Process
Can THYROID problem affect brain development of baby | Dr Reshma Shree | Dr Aravind's IVF
How Thyroid Hormones Support Fetal Brain Development
Thyroid hormones play a crucial role in shaping the fetal brain through a series of intricate biological processes. T4 (thyroxine), a prohormone, crosses into the brain and is converted into T3 (triiodothyronine) by the enzyme type 2 deiodinase (DIO2). This conversion primarily occurs in glial cells and astrocytes, with about 80% of active brain T3 being produced locally.
Once formed, T3 binds to nuclear receptors TRα1 and TRβ1, regulating approximately 5% of neural cell genes. These genes influence diverse processes, including cell cycle progression (via proteins like p53 and cyclins) and the organization of a cell's internal structure (such as actin and microtubule dynamics).
"T4 is essential for fetal neurogenesis, highlighting the importance of adequate treatment for mothers with hypothyroidism." - Federico Salas-Lucia et al., Researchers
These molecular activities lay the groundwork for thyroid hormones' role in guiding neural cell differentiation.
Neural Cell Growth and Specialization
Thyroid hormones are key drivers of neural precursor cell (NPC) differentiation, influencing their progression through the cell cycle and determining their final identity - whether they become neurons, oligodendrocytes, or other brain cell types. T3, in particular, directs NPCs along the dorsal projection trajectory, a critical pathway for forming the cerebral cortex.
A 2026 study in JCI Insight highlighted this process using stem cells from a patient with a mutation in the MCT8 transporter, which is responsible for moving thyroid hormones into cells. Researchers found that without proper T4 activation inside cells via the DIO2 enzyme, neural precursor cells failed to differentiate into neurons. This confirmed that intracellular activation of thyroid hormones is essential for normal brain cell development during fetal growth.
Thyroid hormones also regulate myelination, the process of insulating nerve fibers to ensure rapid signal transmission. T3 stimulates oligodendrocytes to mature and produce structural proteins like myelin basic protein (MBP). Without sufficient thyroid hormones, this insulation process is delayed or incomplete, which can disrupt the brain's internal communication system.
With these mechanisms in mind, it’s important to understand the critical timeframes when these processes are most impactful.
Key Periods for Fetal Brain Growth
The first trimester is the most critical phase for brain development. During this time, the fetus relies entirely on maternal T4, as its own thyroid gland doesn’t become functional until around weeks 16–20. By week 10 of gestation, thyroid hormone receptors are already present in the brain, allowing the developing brain to respond to these hormones long before the fetus can produce them independently.
Interruptions in this period can lead to irreversible structural changes. A post-mortem study of an 11-year-old boy with MCT8 deficiency (Allan-Herndon-Dudley syndrome) revealed delayed neocortex development, reduced axon sizes, and the absence of parvalbumin interneurons. This underscores how thyroid hormone deprivation during early development results in permanent structural defects.
Timing is critical because thyroid hormones influence the "inside-out" formation of the neocortex. This process involves new neurons migrating past older ones to establish the brain’s six-layered structure. Thyroid hormones regulate Reelin, a protein secreted by Cajal-Retzius cells that guides these migrating neurons to their correct positions. Without adequate thyroid hormones, this precise architectural process is disrupted, leading to structural abnormalities that cannot be corrected after birth.
Fetal Dependence on Maternal Thyroid Function
Fetal development in early pregnancy is heavily reliant on maternal thyroid hormones. Before the fetal thyroid becomes functional, the fetus depends entirely on the mother’s hormone supply. While the fetal thyroid begins limited hormone production around 11–12 weeks of gestation, it doesn’t fully mature until about 18–22 weeks. Even after this point, maternal thyroxine (T4) continues to play a significant role. For instance, in newborns with thyroid agenesis (where the thyroid gland is entirely absent), cord blood T4 levels are still 30% to 50% of normal concentrations, highlighting the importance of maternal hormone transfer.
Pregnancy also demands an increase in maternal thyroid hormone production and iodine intake - rising by 50–75% to meet both maternal and fetal needs.
How Thyroid Hormones Cross the Placenta
The transfer of thyroid hormones from mother to fetus is a carefully regulated process. Maternal T4 is the primary hormone that crosses the placenta, facilitated by specific transmembrane transporters like monocarboxylate transporter 8 (MCT8), MCT10, and organic anion transporting polypeptides (OATPs). These transporters ensure the hormones move efficiently from the maternal bloodstream into the fetal circulation.
The placenta doesn’t allow hormones to pass freely. Instead, it actively controls the transfer to protect the fetus. For example, the placenta contains high levels of type 3 deiodinase (D3), an enzyme that deactivates T4 and T3 to prevent overexposure while recycling iodine for fetal use.
"Transfer of T4 from the mother to the fetus protects the fetal brain in congenital hypothyroidism, preventing neurological damage before birth, and making it possible that early postnatal treatment is effective." - Juan Bernal, MD, PhD
By gestational week 5–6, small amounts of maternal T4 can already be detected in the coelomic fluid surrounding the embryo. This is a crucial milestone, as brain development begins early in pregnancy. Once T4 crosses the placenta, it interacts with specific receptors in the fetal brain to support its development.
Thyroid Hormone Receptors in the Fetal Brain
Thyroid hormone receptors (TRs) are present in the fetal brain as early as 7 to 8 weeks of gestation, long before the fetus can produce its own hormones. The enzyme type 2 deiodinase (DIO2), which converts T4 into its active form, triiodothyronine (T3), is expressed in the cerebral cortex by week 7, preparing the brain to utilize maternal hormones.
Among these receptors, TRα1 is the most abundant, making up 70% to 80% of thyroid receptor expression. These receptors act like "molecular switches." When maternal T3 binds to them, they activate genes essential for brain development. Without sufficient maternal T3 - such as in cases of maternal hypothyroidism - these receptors can suppress the very genes needed for proper brain formation, potentially leading to developmental issues.
Effects of Low Maternal Thyroid Hormones
When thyroid hormone levels in pregnant mothers drop too low, the consequences for fetal brain development can be profound and long-lasting. These hormones play a critical role in shaping the fetal brain, and insufficient levels can disrupt key developmental processes. The result? Structural changes in the brain that often lead to measurable cognitive and behavioral challenges.
Changes to Brain Structure and Function
Low maternal thyroid hormone levels can significantly alter the developing brain's structure. One major issue is how these deficiencies interfere with the formation of the neocortex, the brain's outer layer responsible for higher-order functions. Normally, neurons migrate in an orderly fashion to create the six-layered neocortex. However, a lack of thyroid hormones reduces the production of Reelin, a protein that guides neurons to their correct positions. Without enough Reelin, neurons often end up misplaced - a condition called neuronal ectopia - resulting in a disorganized brain structure.
A 2016 study conducted at The Hospital for Sick Children (SickKids) in Toronto examined MRI scans of 22 children, ages 10–12, whose mothers were treated for hypothyroidism during pregnancy. The findings were striking: cortical thinning in the left fusiform gyrus and right precuneus, areas crucial for language and spatial awareness. At the same time, some brain regions, like the superior frontal and inferior occipital areas, showed thickening, creating an abnormal developmental pattern. Researchers also discovered that maternal TSH levels during pregnancy directly correlated with the extent of thinning in frontal and temporal brain regions.
Other areas of the brain, such as the hippocampus, cerebellum, and corpus callosum, also show signs of damage. These include reduced volume, disrupted white matter tracts, decreased myelination, and stunted growth of dendrites and axons in key types of brain cells, such as Purkinje and pyramidal cells.
"Thyroid hormone deficiency during critical transition periods may lead to irreversible brain damage, the consequences of which depend on the severity and duration of the deficiency, and most importantly its time of onset." - Juan Bernal, MD, PhD, Sols-Morreale Institute for Biomedical Research
Cognitive and Behavioral Effects in Children
The structural changes caused by low maternal thyroid hormones have real-world consequences for children. Cognitive and behavioral challenges often emerge early in life. For example, children born to mothers with first-trimester free T4 levels below the 10th percentile face a 1.5 to 2 times higher risk of cognitive delays by age three. By age six, these children score an average of 4.3 points lower on IQ tests compared to their peers whose mothers had normal thyroid levels.
The Generation R Study, based in the Netherlands, tracked 3,659 mother-child pairs and highlighted the long-term effects of this deficiency. By age six, children of mothers with low free T4 levels during early pregnancy scored lower on non-verbal IQ tests. By age eight, MRI scans revealed reduced total gray matter and cortical volume, confirming that these early hormonal deficiencies caused permanent brain changes.
These children are also more likely to experience Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), and expressive language delays. Reduced hippocampal volume affects verbal memory, while abnormalities in the corpus callosum contribute to problems with attention-shifting and vocabulary development.
"Maternal hypothyroxinemia represents a paradigm of how an innocuous and asymptomatic endocrine maternal status, hardly diagnosed in clinical practice, can have a lifetime impact on the fetus intellectual performance." - Alexandra Miranda and Nuno Sousa, University of Minho
In severe cases, when both mother and fetus lack adequate thyroid hormones, infants may develop neurological cretinism, a condition marked by profound intellectual disability. Even mild or subclinical thyroid deficiencies can negatively impact a child's neuropsychological development, highlighting the importance of early detection and treatment.
Maintaining Healthy Thyroid Levels During Pregnancy
Keeping thyroid levels in check during pregnancy is vital for your baby's brain development. The tricky part? Striking the right balance. Too little thyroid hormone can lead to developmental issues, while too much can be equally damaging. This delicate equilibrium requires regular monitoring and, when needed, precise adjustments.
The U-Shaped Relationship Between Thyroid Levels and IQ
Thyroid hormone levels during pregnancy follow a U-shaped curve when it comes to their impact on a child's IQ. Both high and low levels can negatively affect brain development and cognitive abilities.
Overt hypothyroidism (high TSH and low free T4) affects about 0.3% to 0.5% of pregnancies, while overt hyperthyroidism (low TSH and high free T4) occurs in roughly 0.1% to 0.4%. Both conditions are linked to lower IQ scores and developmental challenges in children. This highlights the importance of keeping thyroid levels within the optimal range for healthy brain development.
Testing and Managing Thyroid Health
Pregnancy changes how your thyroid functions. TSH levels vary by trimester, partly because human chorionic gonadotropin (hCG) suppresses TSH in early pregnancy. For example, during the first trimester, 15% of healthy pregnant women have TSH levels below the non-pregnant lower limit of 0.4 mU/L due to normal physiological shifts.
Trimester | TSH Range by Trimester |
|---|---|
First Trimester | 0.1 – 2.5 mIU/L |
Second Trimester | 0.2 – 3.0 mIU/L |
Third Trimester | 0.3 – 3.0 mIU/L |
Women with thyroid conditions should test every 4–6 weeks early in pregnancy and once after 30 weeks. If you're on levothyroxine, contact your doctor right away after a positive pregnancy test. Dosage typically needs to increase by 30% to 50%, often by adding two extra doses per week. This adjustment ensures your body can produce the approximately 50% more thyroid hormone needed during pregnancy to support both you and your baby.
Daily iodine intake is also essential - pregnant women need 250 mcg per day. You can meet this requirement through a combination of iodine-rich foods (like dairy, seafood, and iodized salt) and prenatal vitamins containing 150 mcg of iodine as potassium iodide. But be cautious: too much iodine (over 500 mcg daily) can lead to fetal goiter and hypothyroidism.
"Thyroid hormones are crucial for normal development of your baby's brain and nervous system. During the first trimester... your baby depends on your supply of thyroid hormone." - NIDDK
For women managing hypothyroidism, the goal is to keep TSH levels in the lower half of the trimester-specific range. Levothyroxine (T4) is the preferred treatment because it crosses the placenta and converts to T3 in the fetal brain. Medications containing T3, like desiccated animal thyroid, are not recommended since T3 doesn't reach the fetal brain as effectively. These strategies help ensure your baby's brain develops properly throughout pregnancy.
Recent Research on Thyroid Hormones and Brain Development
Scientists are leveraging cutting-edge laboratory models to uncover how thyroid hormones influence fetal brain development. These new techniques are shedding light on molecular pathways that were previously out of reach in human studies. The findings are offering a clearer picture of how maternal thyroid hormones play a role in shaping the fetal brain.
Findings from Stem Cell and Organoid Studies
Researchers have developed human brain organoids - tiny 3D brain structures - that replicate the development of the fetal brain during the first trimester (weeks 6.5 to 14). These models allow scientists to observe critical developmental processes that were once impossible to study directly.
In a 2025 study published in JCI Insight, Dr. Federico Salas-Lucia and his team at the University of Chicago used stem cells from a 6-year-old boy with Allan-Herndon-Dudley Syndrome to create cortical organoids aged 50 days. Their research showed that when the MCT8 transporter is impaired, neural precursor cells struggle to mature into neurons, leading to a 90% decrease in projection neurons compared to healthy controls. However, supplementing with T3 (60 nM) partially restored neuronal differentiation.
"Growing brain organoids require TH, which is critical for human neurogenesis and oligodendrogenesis. These models have proven useful in screening drugs with potential therapeutic effects for patients with genetic thyroid diseases." - Federico Salas-Lucia, PhD, University of Chicago
The study also revealed that neural precursor cells convert T4 into T3 using the enzyme DIO2. This intracellular process is essential for activating genetic programs that guide whether cells continue dividing or differentiate into neurons. In control cells, DIO2 activity was measured at 390 ± 140 pmol/mg T3/h, while MCT8-deficient cells showed only about 4% of that activity (37 ± 14 pmol/mg/h). These findings underline the importance of local T3 activation in early brain development.
Future Research Directions
Building on these discoveries, scientists are exploring new therapeutic targets and mechanisms. A significant shift in thyroid research is the move toward human-specific models, which address the limitations of rodent studies. Human brains differ in structure and thyroid hormone transporter expression, making rodent models less effective for certain aspects of thyroid research. For example, the human blood-brain barrier lacks the OATP1C1 transporter found in rodents, making human brains more susceptible to specific thyroid transport defects.
These human organoid platforms are now being used to screen drugs that can bypass transport defects and address neurological challenges in genetic thyroid disorders. Researchers are also investigating how maternal thyroid levels, which follow a U-shaped relationship with child IQ, affect brain development at the cellular level. Additionally, they are studying the influence of environmental endocrine disruptors on these pathways. Such advancements could pave the way for targeted treatments for pregnant women with thyroid issues, helping to ensure healthy brain development for their children.
Conclusion
Thyroid hormones play a crucial role in fetal brain development. Until the fetal thyroid becomes active around weeks 16–20, the fetus depends entirely on maternal thyroid hormones. Even after activation, maternal hormones continue to supply 30–60% of the fetus’s T4 needs until birth. These hormones are essential for processes like neurogenesis, neuronal migration, myelination, and synaptic formation - key elements for cognitive development.
Maintaining proper thyroid function during pregnancy is essential. Research shows that children born to mothers with first-trimester free T4 levels below the 10th percentile have a 1.5–2× higher risk of cognitive difficulties by age three. Additionally, maternal hypothyroxinemia has been associated with a 4.3-point reduction in IQ by age six. Early thyroid monitoring and care can help prevent these outcomes. Ensuring sufficient iodine intake - an additional 150 μg daily during pregnancy - along with proper thyroid management, can make a significant difference.
"Maternal serum free T4 levels in the first trimester of pregnancy are the major determinant of postnatal psychomotor development." - Alexandra Miranda, Department of Obstetrics and Gynecology, Hospital de Braga
Take control of your thyroid health early in pregnancy. Regular thyroid function tests and adjusting iodine intake to meet increased needs (50–75% higher during pregnancy) can help ensure a healthy pregnancy. Whether through diet or supplements, meeting the recommended iodine intake is critical. Consult your healthcare provider to monitor trimester-specific TSH levels, as TSH naturally decreases in early pregnancy.
For additional guidance on managing thyroid health and overall prenatal care, explore Rita's Pregnancy 101. This resource offers live expert-led sessions, trimester-specific advice, and ongoing online support. With features like prenatal yoga, meditation, and educational workshops, it provides tools to support your physical and mental well-being throughout this important journey.
FAQs
How can I make sure I’m getting enough iodine during pregnancy?
Getting enough iodine while you're pregnant plays a key role in your baby's brain development. Early on, your baby depends entirely on your thyroid hormones, which need iodine to function properly. To keep your iodine levels where they should be, make sure to include iodine-rich foods in your meals. Some great options are iodized salt, fish, dairy products, and seaweed.
In the U.S., most people meet their iodine needs through iodized salt and a varied diet. But if you have dietary restrictions or avoid seafood, it’s worth having a conversation with your healthcare provider. They can help you figure out if an iodine supplement might be necessary. Keeping an eye on your iodine intake and thyroid health during pregnancy is crucial, as low levels could lead to hypothyroidism, which may affect your baby’s development.
By eating a balanced diet and working with your doctor, you can ensure you're supporting your baby’s growth and development in the best way possible.
What are the symptoms of low thyroid hormone levels during pregnancy?
Low thyroid hormone levels during pregnancy, referred to as hypothyroidism, can lead to various symptoms. These may include feeling unusually tired, low mood, a heightened sensitivity to cold, swelling in the face, unexplained weight gain, and difficulty with bowel movements. Changes in skin and hair are also common, such as dry or flaky skin and hair thinning or even noticeable hair loss.
If you’re dealing with any of these issues, it’s essential to consult your healthcare provider. Thyroid health is crucial not just for your own well-being but also for your baby’s healthy development.
What role do thyroid hormones play in a baby’s brain development during pregnancy?
Thyroid hormones play a crucial role in a baby’s brain development during pregnancy. They support essential processes like neurogenesis (the creation of new brain cells), brain maturation, and the growth of the cerebral cortex, which is responsible for cognitive and motor skills.
In the early stages of pregnancy, a baby is entirely dependent on the mother’s thyroid hormones. If the mother has hypothyroidism (low thyroid hormone levels), it can affect the baby’s brain development, possibly leading to delays in growth and learning abilities. Regular prenatal checkups to monitor thyroid hormone levels are key to promoting healthy brain development for your baby.
